Prognostic Significance of Morphotypes in Canine Lymphomas a Systematic Review of Literature

Evaluation of the Proliferative Activity of Diffuse Big B-Prison cell Lymphoma (DLBCL) in Dogs with Respect to Patient Eligibility for Anthracycline-Based Chemotherapy
one
Veterinary Diagnostic Laboratory VetDiagnostyka, 20-418 Lublin, Poland
ii
Sub-Section of Pathomorphology and Forensic Veterinary Medicine, Department and Clinic of Brute Internal Diseases, University of Life Sciences in Lublin, xx-601 Lublin, Poland
3
Regional Veterinary Inspectorate in Opole, Regional Veterinary Laboratory, 45-836 Opole, Poland
*
Author to whom correspondence should be addressed.
Academic Editors: Maria Pia Franciosini and Cinzia Benazzi
Received: 27 February 2021 / Revised: 12 April 2021 / Accustomed: 17 April 2021 / Published: 20 April 2021
Unproblematic Summary
Canine lymphomas commonly have ambitious behavior, only respond well to chemotherapy. Despite proper diagnosis and numerous available therapeutic regimens, information technology is difficult to determine the prognosis and choose the optimal method of treatment in each individual patient. Lengthened large B-cell lymphoma is the most normally diagnosed histological subtype of canine lymphoma and is treated with anthracyclines alone or in combination with other chemotherapeutics. A new diagnostic marker of prognostic and predictive value is topoisomerase IIα, which too constitutes a molecular target for anti-cancer drugs belonging to the grouping of topoisomerase IIα inhibitors including anthracyclines. Proliferative activity was estimated in samples of enlarged lymph nodes in dogs with diffuse large B-prison cell lymphoma based on mitotic count and immunohistochemical evaluation of topoisomerase IIα and Ki67 antigen expression with a view to qualifying patients for anthracycline-base chemotherapy. It has been shown that higher levels of topoisomerase IIα expression corresponded to a higher mitotic count but not to Ki67 index. These results betoken that an immunohistochemical evaluation of topoisomerase IIα expression can be used to develop a diagnostic-clinical protocol for the handling of dogs with diffuse large B-cell lymphoma using anthracycline-based chemotherapy.
Abstract
Unlike types of canine lymphoma respond differently to chemotherapy and have different prognoses. Diffuse large B-prison cell lymphoma (DLBCL) is the nearly common lymphoma in dogs. Topoisomerase II alpha (TOPIIα) protein has been shown to be a proliferation marker associated with prognostic significance. The aim of the study was to determine the human relationship betwixt TOPIIα expression, mitotic count (MC), and Ki67 antigen index in DLBCL in dogs, taking into account the applicability of these parameters to select the chemotherapy protocol with emphasis on the employ of anthracycline drugs. Samples of formalin-fixed paraffin-embedded lymph nodes from 34 dogs with DLBCL were immunohistochemically labelled with anti-TOPIIα and Ki67. The number of positive cells and the intensity of the reaction were taken into account in order to assess TOPIIα expression. MC was estimated in the hematoxylin and eosin-stained slides in the area of ii.37 mm2. Positive clan betwixt TOPIIα and MC, just no clan betwixt TOPIIα and Ki67 was found. Information technology can exist concluded that the immunohistochemical decision of TOPIIα every bit a molecular target for drugs from the anthracycline grouping may be used in association with MC to establish a diagnostic-clinical protocol for selecting dogs with DLBCL for handling with anthracycline drugs.
ane. Introduction
Non-Hodgkin lymphomas are the most frequently diagnosed neoplasms of the hematopoietic system in dogs. They are ranked tertiary amongst the most prevalent mammary and peel tumors [1,2,iii]. The well-nigh commonly diagnosed histological subtype of canine lymphoma is diffuse large B-cell lymphoma (DLBCL) [4,five,6,seven,8,9]. Almost cases of canine lymphoma are diagnosed in clinical stage III, 4, or V, according to WHO criteria [10,eleven]. The basic diagnostic process in the case of suspected lymphoma is the cytological examination of the fine-needle aspirates from neoplastic lymph nodes. According to WHO guidelines, histopathological examination of a surgically removed lymph node supplemented with immunophenotyping and polymerase chain reaction (PCR) for antigen receptor rearrangement (PARR) is useful to definitively diagnose, plant prognosis, and in some cases guide treatment options [12,13,fourteen,fifteen]. The well-nigh common method for assessing the lymphoma phenotype is immunohistochemistry, while, in guild to appraise the malignancy and prognosis of the affliction progression, cell proliferation indicators are taken into account, including the mitotic count (MC) and the cess of the expression of the cell bike dependent Ki67 protein [eleven,xv,16,17]. Lymphomas are neoplasms with loftier chemosensitivity [eighteen,19,20]. The chemotherapy of canine malignant lymphoma involves single or multi-drug protocols which allow for complete or partial remission and, in some cases, can extend a domestic dog's life up to 36 months [21,22,23,24,25]. At the aforementioned fourth dimension, despite confirmed diagnosis and numerous bachelor therapeutic regimens, it is difficult to decide the prognosis, choose the optimal method of treatment, and predict the response to handling in each individual patient. Due to the fact that most cytostatic drugs bear upon actively dividing cells, the bones indicator of tumor chemosensitivity is the determination of the fraction of cells going through the cell bike. Moreover, the information on proliferative activity allows prediction of the tumor'south aggressiveness and identification of cases with the prognoses of a quick relapse or the gamble of metastasis. The side effects of chemotherapy drugs, such as nephrotoxicity, hepatotoxicity, and cardiotoxicity, as well as differentiated response to handling, imply the demand for a conscientious cess of tumor cells' susceptibility to treatment. Counting of cells demonstrating mitotic figures and the assessment of the expression of regulatory proteins, such as Ki67, is nigh commonly used to assess the proliferative activeness of neoplasms [17,26]. A new proliferation marker of prognostic and predictive value is topoisomerase IIα (TOPIIα), which also constitutes a molecular target for some anti-cancer drugs belonging to the group of TOPIIα inhibitors. Topoisomerases are members of the family of enzymes involved in DNA metabolism, including transcription, recombination, replication, and chromosome segregation during cell division. TOPIIα is associated with the segregation of the newly replicated chromosome pairs, condensation and germination of the chromosome framework, and modification of the DNA superhelix [27,28,29,30]. TOPIIα expression is the highest in the G2/Thou phases of the cell cycle, and the lowest at the end of mitosis. The largest group of cytostatic drugs affecting this protein are anthracyclines, which, due to relatively good therapeutic effects, moderate adverse reactions, and low cost, are commonly used in veterinary oncology. The nearly commonly used drugs belonging to this group are doxorubicin and epirubicin [31,32,33,34,35]. These drugs create a cleavable complex consisting of the drug, TOPIIα, and DNA strand, blocking relegation and enzyme release, leaving the DNA with a permanent double strand intermission leading to prison cell apoptosis [thirty].
The aim of the report was to determine the relationship between TOPIIα expression and MC and Ki67 antigen alphabetize in DLBCL in dogs, taking into account the applicability of the determined parameters to found the optimal chemotherapy protocol with accent on the use of anthracycline drugs.
ii. Material and Methods
2.1. Material for the Report
The textile for the study consisted of samples of enlarged popliteal lymph nodes surgically removed from 34 selected dogs of varied sexual activity and age, with newly diagnosed, previously untreated lymphoma admitted to the clinics of Faculty of Veterinary Medicine in Lublin betwixt January 2013 and December 2017. To be eligible for the study, dogs had to undergo a consummate diagnostic evaluation, including history and physical test, chest X-ray, and ultrasound of the abdominal crenel, as well equally pathomorphological examination indicating the diagnosis of DLBCL. The surgical procedure with the use of propofol (Scanofol x/mL, ScanVet, Gniezno, Poland) for anesthesia of dogs was performed according to the applicable rules, after obtaining the written consent of the owner.
ii.2. Histology and Immunohistochemistry
Sections of lymph nodes were fixed in 10% buffered formalin with pH = 7.two for 24 h and, later on existence dewaxed and rehydrated through a series of graded booze solutions in a tissue processor (Leica TP-xx), were embedded in paraffin blocks. The 4 µm tissue sections were stained with hematoxylin and eosin (HE). Histologic classification followed the WHO criteria [vi]. For immunohistochemical test, the streptavidin–biotin–peroxidase complex technique was used (LSAB plus, horseradish peroxidase -HRP, K0690, Dako, Glostrup, Kingdom of denmark). The lymphoma phenotype was determined using CD3 and CD79α antibodies. The cell proliferation activity was determined using Ki67 and TOPIIα antibodies. The primary antibodies used in the report are summarized in Tabular array 1.
Dewaxed and rehydrated sections were subjected to antigen retrieval. Target antigen retrieval for CD3 was performed by immersion in proteinase One thousand for x min, whereas heat-induced epitope retrieval for CD79α, Ki67, and TOPIIα were performed by an automatic pressure cooker in a citrate buffer of pH 6.0. The enzyme labelling the reaction site was horseradish peroxidase conjugated with streptavidin. Tetrahydrochloride-three,-3-diaminobenzidine (DAB) was used as a chromogen (SK-4100; Vector Laboratories, Peterborough, United kingdom). The sections were counterstained with Mayer's hematoxylin and covered in PERTEX (Histolab). For each analysis, a double control system was used, i.e., method control and negative command. In the negative controls, the incubation with the master antibody was replaced by incubation with mouse IgG serum under the same weather of fourth dimension and temperature. The positive control was the healthy unchanged tissue from canine tonsil. At each stage, two contained pathologists performed the assessment of the histopathological preparations and immunohistochemical reactions. A computer-assisted microscopic prototype analysis arrangement was used to quantify the immunohistochemical parameters. The arrangement included a calorie-free microscope (Nikon Eclipse E-600, Nikon Instruments, Tokyo, Japan) coupled with a digital photographic camera (Nikon DS-Fi1, Nikon Instruments, Tokyo Japan) and a PC with image analysis software (NIS-Elements BR-ii.xx, Laboratory Imaging, Praha, Czech republic). Lymphoma phenotype was determined by the pct of expression of CD3 and CD79α molecules (membrane and cytoplasmic reaction) in neoplastic cells in x fields of view under 40x magnification (in the surface area of 2.37 mm2). B-cell lymphomas were diagnosed when more than eighty% of cells expressed the CD79α antigen. T-cell lymphomas were excluded from the study. When evaluating the expression of the Ki67 antigen at 40x magnification, an index was calculated using the pct of positive cells in 500 tumor cells. To appraise TOPIIα expression, the scoring system proposed by Remmele et al. was used to evaluate the expression of estrogen receptors [36]. Based on the assay of no less than 500 cells at 40x magnification, the number of stained cells was estimated and assigned a score, where 0 points corresponded to no positive cells, 1 betoken to 25% positive cells, 2 points to 26–50% positive cells, 3 points to 51–75% positive cells, and 4 points to over 75% positive; and the intensity of their staining, where 0 points—no reaction, 1 point—low staining intensity, 2 points—medium staining intensity, and 3 points—potent staining intensity. The final outcome was the product of points obtained from the assessment of both parameters, where 0 points meant no expression (-), 1 point meant very low expression (very low "+"), 2–4 points meant low expression ("++"), v–seven meant medium expression ("+++"), and 8–12 ("++++") meant high TOPIIα expression. MC was assessed in hematoxylin and eosin-stained sections by counting cells demonstrating mitotic figures in ten fields of view at 40x magnification (in the area of 2.37 mmii).
2.3. Statistical Analysis
The obtained results were subjected to statistical analysis. The values of the measurable parameters were presented past means of the hateful, median, lower and upper quartile, minimum and maximum values, and standard deviation; and for non-measurable ones, the number and percentage. The normality of the distribution of variables in the studied groups was checked using the Shapiro–Wilk normality test. The Student's t-exam was used to test the differences betwixt the two groups, and in the case of failure to meet the conditions of its awarding, the Mann–Whitney test. Spearman'south rank correlation was used to appraise the relationship between some of the variables. The level of significance was gear up at p < 0.05, indicating the beingness of statistically significant differences or relationships. The database and statistical inquiry were carried out on the basis of the Statistica nine.i calculator program (StatSoft, Cracow, Poland).
iii. Results
Thirty-four dogs with DLBCL that met the admission criteria out of 67 dogs with diagnosed lymphoma were enrolled in the report. The dogs included in the study were ii.5 to thirteen years old (median half-dozen years). The grouping consisted of 18 males (53%) and 16 females (47%). Mixed breed dogs: 10 (29.4%); German shepherd: 9 (26.5%); and Gold retriever: four (11.8%) were the about common breeds in this study. Phase III clinical progression was found in 20 dogs and phase IV in 14 dogs. Information regarding signalment, age, sex, breed, and stage of disease are presented in Table 2.
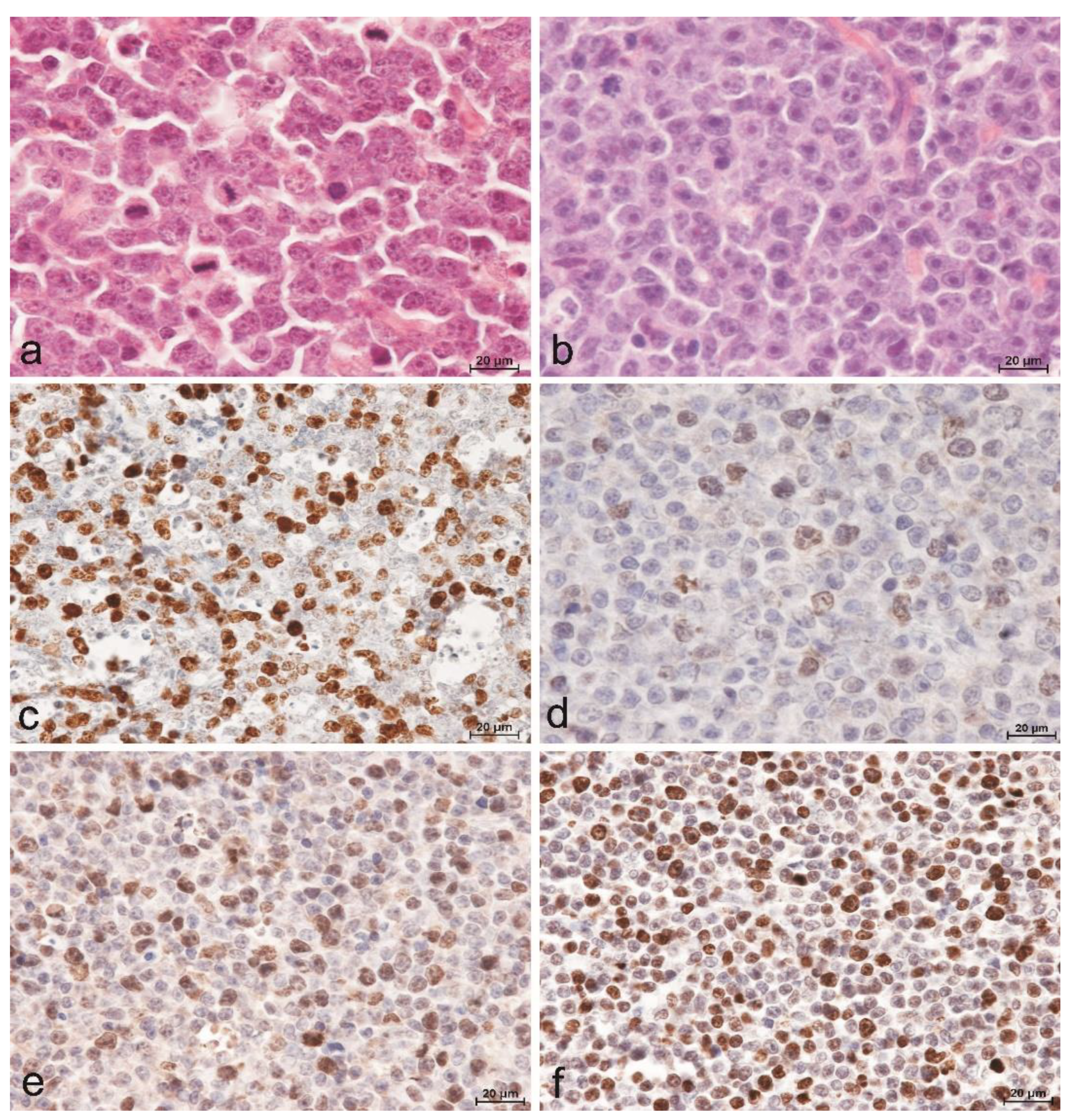
The diagnosis of DLBCL was fabricated by evaluating cytological and histomorphological features in conjunction with immunohistochemical analysis to detect expression of CD79α and CD3 by the tumor cells. All dogs included in this study had an expression level of CD79α exceeding lxxx% of tumor cells, while the level of CD3 expression was less than xx% of tumor cells. The hallmark histomorphologic feature used to diagnose DLBCL was lengthened effacement of nodal architecture by sheets of neoplastic B-cells with scanty cytoplasm and predominantly large nuclei (≥two red blood cells in diameter). The nuclei of these cells were round, cleaved, or indented. Twenty-8 (82.4%) dogs were further classified with centroblastic subtype (DLBCL-CB) based on the presence of multiple nucleoli (Figure 1a), while six (17.half-dozen%) dogs were classified with immunoblastic subtype (DLBCL-IB) based on dominance of a single central prominent nucleolus (Figure 1b). A positive reaction for the presence of Ki67 antigen (Figure 1c) and TOPIIα were evident in all cases with a diffuse granular nuclear design. Although in the case of TOPIIα, the variability in the reaction intensity and the number of positive cells were demonstrated (Figure 1d–f).
The value of the Ki67 antigen index ranged from 23.8% to 72.three%. The median value of Ki67 antigen index in the DLBCL-IB grouping was college (41.viii%) than in the group of DLBCL-CB (38.1%) with no statistical difference (Table iii). Higher expression of TOPIIα was observed at the periphery of the examined lymph nodes. The areas with the highest expression were selected for the cess. Based on the established TOPIIα expression scoring system, the nearly numerous were cases with moderate TOPIIα expression (13 cases, 38.ii%), followed past stiff expression (9 cases, 26.5%). The total number of lymphomas with moderate to loftier expression of TOPIIα was 22, which deemed for 64.vii% of cases in the study population. DLBCL with very low or depression TOPIIα expression included 12 cases (35.3%) (Table 4). At that place was no statistically significant difference in TOPIIα expression betwixt DLBCL-CB and DLBCL-IB lymphomas. In both groups, most cases were characterized with a moderate level of expression and deemed for 35.71% and 50%, respectively. The MC value ranged from ix to 135 (median value = 24.5), with the value in DLBCL-CB grouping being significantly higher (median value = 29.5) compared to the DLBCL-IB group (median value = 15.0; U = 11.5; p < 0.001) (Table 3).
In the conducted study, it was found that higher MC values corresponded to higher Ki67 antigen index values, and the correlation was statistically meaning (R = 0.373; p = 0.030) (Figure 2). Similarly, college levels of TOPIIα expression corresponded to higher MC values (R = 0.400; p = 0.019) (Effigy 3). Nevertheless, no statistically pregnant correlation was found between the level of TOPIIα expression and the Ki67 values (R = 0.053; p = 0.764) (Figure 4).
For the purposes of statistical assay, lymphomas with very depression and low TOPIIα expression (12 cases) were grouped, as well as lymphomas with moderate and high TOPIIα expression (22 cases). At that place were no statistically meaning differences in the Ki67 and MC values between the group of lymphomas with very depression and depression TOPIIα expression and the group with moderate and high TOPIIα expression (Table 3).
iv. Discussion
The aim of the study was to assess the cellular proliferation in DLBCL in dogs based on the expression of TOPIIα and Ki-67 antigen, and to determine the MC value in terms of patient qualification for chemotherapy with anthracyclines. DLBCL is the nearly commonly diagnosed lymphoma in dogs, accounting for approximately xxx% of all lymphomas found in this species, nearly of which are the centroblastic subtype (DLBCL-CB), while the immunoblastic subtype (DLBCL-IB) is less prevalent [iv,11,37]. This correlation was confirmed in the conducted study. In the vast majority of cases, the illness occurred in middle-aged (half-dozen years onetime) large breed dogs (88%), about of which were mixed breed (29.4%), German Shepherd (26.5%), or Golden Retriever (11.eight%). Most of the studies did not prove a significant influence of brood, historic period, and gender on the gamble of lymphoma development or on the course of the disease and prognosis regarding survival time and response to treatment [33,38]. Although ane of the studies demonstrated increased susceptibility to the occurrence of lymphoma in unsterilized females and males, it has non been unequivocally confirmed in other studies [33]. In European countries, the breeds predisposed to lymphoma include Doberman, Rottweiler, Boxer, and Bernese mount dogs, where boxers tended to develop T-prison cell lymphomas while Rottweilers had a high prevalence of B-jail cell lymphomas [39]. In our study, mixed-brood and German Shepherd dogs prevailed, which is undoubtedly related to the limerick of the dog population in the region the animals came from and reflects the differences in the results of epidemiological studies [37,39]. Apart from the histological blazon and immunophenotype, the parameters determining the proliferative activity of cells, i.e., MC and Ki67 antigen alphabetize are the most frequently mentioned among the important prognostic factors in lymphomas in dogs. Despite the limitation of the study to only one type of lymphoma, the obtained results testify considerable variation in the MC value and Ki67 antigen index in individual animals. The median MC value was 24.5 (ix–135); whereby, in individuals with the centroblastic subtype, it was significantly higher (median = 29.v) than in those with the immunoblastic subtype (median = 15.0). The obtained hateful values of MC are higher than those presented in the studies conducted by Valli et al. (DLBCL-CB 15.4; DLBCL-IB thirteen.eight) [6]. The median Ki67 antigen index value was 38.5%. There was no statistically pregnant divergence in either the median values of the Ki67 index, nor the level of TOPIIα expression between the corresponding subtypes of lymphoma (Table iii). The conducted study demonstrated a correlation between MC and Ki67 (p = 0.030) in the DLBCL group. Individuals with a college MC value had a higher Ki67 antigen index. This correlation is due to the fact that the expression of the Ki67 antigen was constitute in all cells undergoing the cell wheel, so with the increase in the number of cells in the mitotic phase, the increase in the Ki67 value seems justified.
Another parameter that could potentially be used to appraise the proliferative action is the level of TOPIIα expression, which is as well a molecular target for cytostatic drugs from the anthracycline group (mainly doxorubicin). Medical studies on the predictive and prognostic role of TOPIIα expression in humans take been conducted for many years [xl,41,42,43,44,45]. Researchers are especially interested in the expression of this poly peptide in breast cancer in women. [27,28,29,42]. The reason for this interest is the fact that doxorubicin is i of the primary drugs used in the treatment of breast cancer in women [28]. It is surprising, however, that despite the fairly widespread use of doxorubicin in the treatment of diverse types of tumors in animals, including lymphomas, no studies have been carried out to engagement to determine the level of expression of this poly peptide in private tumors. In the conducted studies, the TOPIIα expression was assessed using a proprietary scheme, taking into account both the number of positive cells and the intensity of their staining. This scheme was used due to doubts raised by many authors regarding the lack of a compatible scheme allowing objective assessment and interpretation of the level of TOPIIα expression. The well-nigh commonly expressed problem was in regard to the need to establish the and then-called cut-off bespeak, i.e., determining what level of TOPIIα expression was considered as depression, medium, or loftier. Nearly authors have only described the observed correlations between the TOPIIα expression and response to treatment, without quantifying the level of the expression. Hajduk et al., examining the level of TOPIIα expression in breast cancer cells, assumed that the lack of expression tin can exist confirmed when the number of "positive" cells did not exceed 5%, low expression with half dozen–xxx%, moderate expression with 31–60%, and high expression with more than 60% of stained cells [27]. Pentheroudakis et al., examining the level of TOPIIα expression in DLBCL in humans, estimated that lymphomas with loftier TOPIIα expression are those in which positive cells establish more than eighty% of all cells. The remaining ones are lymphomas with low (less than eighty%) expression of the protein [forty]. In our study, positive expression of TOPIIα was observed in all the lymph node samples, only with varying intensity. Yet, the vast majority of cases were characterized with loftier and medium expression (22 dogs: 64.7%), and cases with low and very weak expression constituted a minority (12 dogs: 35.iii%). In similar studies carried out in people with DLBCL, high expression of TOPIIα was noted in 91% of patients [40]. The studies demonstrated that the level of TOPIIα expression positively correlated with the mitotic alphabetize (p = 0.019), only no meaning correlation was establish between the Ki67 antigen and TOPIIα values.
All the parameters assessed by the authors allow the estimation of the proliferative action of neoplastic cells, simply they have a variable prognostic value regarding the determination of the patient survival time and predictive value in the context of choosing an appropriate therapy regimen. The most frequently used protocol in the treatment of canine lymphoma is CHOP (Cyclophosphamide, Hydroxydaunorubicin, Oncovin, and Prednisolone) [7,20,21,22]. The use of multi-drug protocols is of import and justified, as each drug has an effect on cells at a dissimilar phase of the prison cell cycle. This allows the accomplishment of the best results in the form of complete or partial remission and longer overall survival. This protocol has been found to be most constructive in patients with high-grade lymphomas, i.e., those with high MC. It was demonstrated that the higher the MC value, the shorter the survival time of dogs with DLBCL. At the aforementioned fourth dimension, a higher MC value resulted in meliorate treatment response, including the achievement of complete remission. Therefore, some authors considered MC to exist the main prognostic parameter in canine lymphomas [16]. The part of Ki67 antigen expression as a prognostic gene in canine lymphomas is ambiguous. Kiupel et al. argued that Ki67 assessment has no statistically significant prognostic value in lymphomas in dogs [16]. According to the above-mentioned authors, the Ki67 antigen alphabetize allows the decision of the per centum of cells in the cell bike, but it does not brand it possible to determine the length of this bike, i.e., it does not allow the assessment of the dynamics of the neoplastic process. Positive expression is observed in both cells with a cell bicycle duration of two days and cells with a jail cell cycle duration of 30 days. On the other hand, a written report by Sierra Matiz et al. demonstrated that in the group of dogs with DLBCL characterized with low Ki67 antigen expression, the survival times were significantly longer than in animals with loftier expression of this poly peptide. [17]. According to the same authors, dogs with high Ki67 alphabetize demonstrated a much ameliorate response to treatment than those with low Ki67 antigen activity. The studies showed no meaning differences in survival times and response to treatment in dogs with low and high MC values. The assessment of the prognostic value of TOPIIα expression in animals is the nearly challenging. The reason is the lack of any studies describing the beliefs of this protein in fauna tumors. Therefore, the results obtained in studies conducted in humans should exist considered as a indicate of reference [40,41,42]. TOPIIα belongs to enzymes involved in the processes related to cell partitioning and Dna metabolism [27,28,29,30]. At the same time, TOPIIα is an enzyme that served as a molecular target for cytostatic drugs from the anthracycline group (the most frequently used drug in this group is doxorubicin, which constitutes a part of the multi-drug CHOP regimen used in the treatment of lymphoma) [21,22,23,24]. Its expression reaches its elevation in the G2/M phase, i.e., just before jail cell partition. This explains the statistically significant correlation observed betwixt the level of TOPIIα expression and the MC value in our study, and at the same time indicates that these two parameters may serve as significant prognostic indicators in selecting patients for anthracycline therapy. Nonetheless, the authors of the article did not find any statistically pregnant relationships betwixt the expression of TOPIIα and Ki67, which, combined with the observations of Kiupel et al., indicates the existence of differences in the expression of both proteins in the cell cycle [xvi]. On the other manus, in studies conducted past Pentheroudakis et al. and Korkolopoulou et al. on lymphomas in humans, a positive correlation was found between the expression of TOPIIα and Ki67 [40,41]. Still, in the cited studies, merely the percentage of cells showing a positive reaction was considered, without grading its intensity. Moreover, when comparing the same lymph node samples, information technology was noticed that all TOPIIα expressing cells too expressed Ki67, simply not all Ki67 expressing cells expressed TOPIIα. Thus, it can be assumed that a high level of the Ki67 protein does not always betoken loftier TOPIIα expression. Considering that TOPIIα is a molecular target for drugs from the anthracycline group, information technology can be ended that the differences in poly peptide expression observed in our study in dissimilar individuals with DLBCL causes different responses to handling with TOPIIα inhibitors. The obtained results point potentially greater chemosensitivity of lymphomas with high TOPIIα action to the activity of chemotherapeutic agents being the enzyme inhibitors. The fact that the conducted studies showed a relatively depression degree of correlation between MC and Ki67 antigen alphabetize and between MC and TOPIIα expression, with no correlation between Ki67 antigen alphabetize and TOPIIα expression, indicates that the assessment of TOPIIα expression should be used as a key potential predictive indicator for anthracycline-base therapy. This is reflected in clinical observations in humans. According to Hajduk et al., in patients with high TOPIIα expression, the response to treatment with doxorubicin was ameliorate than in patients with low expression of this protein. Additionally, it was found that the overall survival of patients with depression TOPIIα expression treated with doxorubicin was significantly lower than in the case of patients with high TOPIIα expression [27]. In man DLBCL patients with high TOPIIα expression, a much better response to handling using the CHOP protocol was observed, notwithstanding, it did not improve the patients' overall survival [xl]. The preliminary results of the authors' clinical observations indicate that dogs with DLBCL and with high TOPIIα expression reply better to anthracycline-based therapy, which is manifested by a higher percent of complete remission and a longer remission compared to dogs with DLBCL and low TOPIIα expression. However, before the studies are completed, it would be unjustified to draw whatever conclusions regarding the correlation betwixt TOPIIα expression and the clinical furnishings of anthracycline-based therapy in dogs with DLBCL, despite the initial promising results.
5. Conclusions
The obtained results signal that the immunohistochemical assessment of the TOPIIα expression may exist used to develop a diagnostic clinical protocol for the treatment of dogs with diffuse big B-cell lymphoma using anthracycline-based chemotherapy. The limitations resulting from the small number of patients in our report influences statistical assay, and the lack of standardized compatible schemes for assessing the expression of individual jail cell-cycle proteins imply the need for further studies in this area, in detail, clinical studies on a big population of animals, aimed at confirmation of correlation between the level of TOPIIα expression and the other parameters of proliferative activity, as well as the therapeutic effects of treating patients with TOPIIα inhibitors.
Writer Contributions
Conceptualization, P.K.; information curation, Chiliad.B. and A.B.; formal assay, P.K.; investigation, W.Ł.; methodology, Due west.Ł.; project administration, A.B.; resources, P.1000. and Chiliad.B.; software, A.B.; supervision, West.Ł.; validation, P.K. and K.B.; writing—original draft, Due west.Ł.; writing—review & editing, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no fiscal support for the enquiry, authorship, and/or publication of this commodity.
Institutional Review Board Statement
This study was conducted according to the European Directive 2010/63/UE and the Local Ethics Committee for Animal Testing at the University of Life Sciences in Lublin, Poland (consent No. 17/2013).
Informed Consent Argument
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed in the current study are available from the corresponding authors on reasonable request.
Acknowledgments
The authors would like to thank the owners of the animals and technical staff involved in the care of the dogs included in the study.
Conflicts of Interest
The authors declare no disharmonize of interest.
References
- Grüntzig, K.; Graf, R.; Boo, G.; Guscetti, F.; Hässig, One thousand.; Axhausen, K.Westward.; Fabrikant, Due south.; Welle, Chiliad.; Meier, D.; Folkers, Thou.; et al. Swiss Canine Cancer Registry 1955–2008. Occurrence of the most common neoplasm diagnoses and influence of age, breed, body size, sex and neutering status on tumour evolution. J. Comp. Pathol. 2016, 155, 56–170. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.; Pinkerton, M.; Young, Thousand. Withrow & MacEwen'due south Small Animal Clinical Oncology Hematopoietic Tumours, 5th ed.; Elsevier: St Louis, MO, U.s., 2013; p. 608. [Google Scholar]
- Merlo, D.F.; Rossi, 50.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, 5.; Tanara, K.; et al. Cancer incidence in pet dogs: Findings of the animate being tumor registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef]
- Aresu, L.; Martini, V.; Rossi, F.; Vignoli, M.; Sampaolo, M.; Aricò, A.; Laganga, P.; Pierini, A.; Frayssinet, P.; Mantovani, R.; et al. Canine indolent and ambitious lymphoma: Clinical spectrum with histologic correlation. Vet. Comp. Oncol. 2015, xiii, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, G. Canine lymphoma: A review. Vet. Quart. 2016, 36, 76–104. [Google Scholar] [CrossRef]
- Valli, V.East.; San Myint, M.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Nomenclature of canine malignant lymphomas according to the world wellness organization criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef]
- Curran, K.M.; Schaffer, P.A.; Frank, C.B.; Lana, Southward.East.; Hamil, Fifty.East.; Burton, J.H.; Labadie, J.; Ehrhart, E.J.; Avery, P.R. BCL2 and MYC are expressed at high levels in canine diffuse large B-jail cell lymphoma only are not predictive for issue in dogs treated with CHOP chemotherapy. Vet. Comp. Oncol. 2017, 15, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Rebhun, R.B.; Lana, South.Eastward.; Ehrhart, E.J.; Charles, J.B.; Thamm, D.H. Comparative assay of survivin expression in untreated and relapsed canine lymphoma. J. Vet. Intern. Med. 2008, 22, 989–995. [Google Scholar] [CrossRef]
- Childress, Thousand.O.; Ramos-Vara, J.A.; Ruple, A. Retrospective analysis of factors affecting clinical issue following CHOP-based chemotherapy in dogs with primary nodal diffuse big B-jail cell lymphoma. Vet. Comp. Oncol. 2018, xvi, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ponce, F.; Magnol, J.; Ledieu, D.; Marchal, T.; Turinelli, Five.; Chalvet-Monfray, K.; Fournel-Fleury, C. Prognostic significance of morphological subtypes in canine cancerous lymphomas during chemotherapy. Vet. J. 2004, 167, 158–166. [Google Scholar] [CrossRef]
- Valli, V.E.; Kass, P.H.; San Myint, G.; Scott, F. Canine lymphomas: Association of nomenclature type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, fifty, 738–748. [Google Scholar] [CrossRef]
- Sapierzyński, R.; Kliczkowska-Klarowicz, K.; Jankowska, U.; Jagielski, D. Cytodiagnostics of canine lymphomas—Possibilities and limitations. Politico. J. Vet. Sci. 2016, 19, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Bienzle, D.; Vernau, W. The diagnostic cess of canine lymphoma: Implications for handling. Clin. Lab. Med. 2011, 31, 21–39. [Google Scholar] [CrossRef]
- Sayag, D.; Fournel-Fleury, C.; Ponce, F. Prognostic significance of morphotypes in canine lymphomas: A systematic review of literaturę. Vet. Comp. Oncol. 2018, xvi, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Keller, B.; Grest, P.; Börger, C.T.; Guscetti, F. Validation of tissue microarrays for immunohistochemical analyses of canine lymphomas. J. Vet. Diagn. Investig. 2007, 19, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, Thousand.; Teske, Due east.; Bostock, D. Prognostic factors for treated canine malignant lymphoma. Vet. Pathol. 1999, 36, 292–300. [Google Scholar] [CrossRef]
- Sierra, Chiliad.O.R.; Santilli, J.; Anai, Fifty.A.; Da Silva, Grand.C.Fifty.; Sueiro, F.A. Prognostic Significance of Ki67 and Its Correlation with Mitotic Index in Dogs with Diffuse Large B-Cell Lymphoma Treated with xix-Week CHOP-Based Protocol. J. Vet. Diag. Investig. 2018, thirty, 263–267. [Google Scholar] [CrossRef]
- Legendre, A.M. Treatment of Dogs with Lymphoma: A Piece of work in Progress. J. Vet. Intern. Med. 2007, 21, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Curran, 1000.; Thamm, D.H. Retrospective analysis for treatment of naïve canine multicentric lymphoma with a xv-calendar week, maintenance-free CHOP protocol. Vet. Comp. Oncol. 2016, 1, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Flory, A.B.; Rassnick, K.M.; Erb, H.N.; Garrett, L.D.; Northrup, Northward.C.; Selting, Thousand.A. Evaluation of factors associated with second remission in dogs with lymphoma undergoing retreatment with a cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy protocol: 95 cases (2000–2007). J. Am. Vet. Med. Assoc. 2011, 238, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Daters, A.T.; Mauldin, Chiliad.East.; Mauldin, Thou.Northward.; Brodsky, E.M.; Mail, Grand.Due south. Evaluation of a multidrug chemotherapy protocol with mitoxantrone based maintenance (CHOP-MA) for the treatment of canine lymphoma. Vet. Comp. Oncol. 2009, eight, xi–22. [Google Scholar] [CrossRef]
- Rassnick, One thousand.; Bailey, D.; Malone, E. Comparison betwixt L-CHOP and an L-CHOP protocol with interposed treatments of CCNU and MOPP (50-CHOP-CCNU-MOPP) for lymphoma in dogs. Vet. Comp. Oncol. 2010, eight, 243–253. [Google Scholar] [CrossRef]
- Simon, D.; Nolte, I.; Eberle, N. Treatment of dogs with lymphoma using a 12-week, maintenance-free combination chemotherapy protocol. J. Vet. Intern. Med. 2006, xx, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.; Garrett, L.; Vail, D. Evaluation of a high-dose chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J. Vet. Intern. Med. 2000, 14, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.R.; Couto, C.G.; Wittum, T.E. Factors influencing first remission and survival in 145 dogs with lymphoma: A retrospective study. J. Am. Anim. Hosp. Assoc. 2000, 36, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Sunday, Ten.; Kaufman, P.D. Ki-67: More than a proliferation marking. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Hajduk, M.; Olszewski, Due west.P.; Smietana, A. Evaluation of the predictive value of topoisomerase Ii alpha in patients with breast carcinoma. Pol. J. Pathol. 2009, 60, 115–123. [Google Scholar]
- Sosinska-Mielcarek, K.; Jassem, J. Predictive role of topoisomerase IIα expression in anthracycline based breast cancer chemotherapy. J. Oncol. 2005, 55, 252–256. [Google Scholar]
- Villman, K.; Sjöström, J.; Heikkilä, R.; Hultborn, R.; Malmström, P.; Bengtsson, Due north.O. TOP2A and HER2 gene amplification equally predictors of response to anthracycline treatment in breast cancer. Acta Oncol. 2006, 45, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.Fifty. Targeting Deoxyribonucleic acid topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, ix, 338–350. [Google Scholar] [CrossRef]
- Al-Nadaf, S.; Rebhun, R.B.; Curran, M.Thou. Retrospective assay of doxorubicin and prednisone as offset-line therapy for canine B-cell lymphoma. BMC Vet. Res. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Beaver, 50.; Strottner, G.; Klein, M. Response rate after administration of a unmarried dose of doxorubicin in dogs with B-cell or T-cell lymphoma: 41 cases (2006–2008). J. Am. Vet. Med. Assoc. 2010, 237, 1052–1055. [Google Scholar] [CrossRef]
- Keller, Due east.T.; MacEwen, Due east.G.; Rosenthal, R.C.; Helfand, S.C.; Trick, L.E. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J. Vet. Intern. Med. 1993, seven, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lori, J.C.; Stein, T.J.; Thamm, D.H. Doxorubicin and cyclophosphamide for the treatment of canine lymphoma: A randomized, placebo-controlled report. Vet. Comp. Oncol. 2010, 8, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.W.; Cripps, P.; Marrington, A.1000. Epirubicin as part of a multi-agent chemotherapy protocol for canine lymphoma. Vet. Comp. Oncol. 2013, 11, 185–198. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.Due east. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Ernst, T.; Kessler, M.; Lautscham, Eastward. Multicentric lymphoma in 411 dogs—An epidemiological study. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere. 2016, 44, 245–251. [Google Scholar]
- Villamil, J.A.; Henry, J.C.; Hahn, W.A. Hormonal and sex impact on the epidemiology of canine lymphoma. J. Cancer Epidemiol. 2009, 2009, 591753. [Google Scholar] [CrossRef]
- Comazzi, S.; Marelli, S.; Cozzi, M. Breed-associated risks for developing canine lymphoma differ among countries: An European canine lymphoma network study. BMC Vet. Res. 2018, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Pentheroudakis, 1000.; Goussia, A.; Voulgaris, E.; Nikolaidis, Yard.; Ioannidou, E.; Papoudou-Bai, A. High levels of topoisomerase IIα protein expression in diffuse big B-prison cell lymphoma are associated with high proliferation, germinal centre immunophenotype, and response to treatment. Leuk. Lymphoma 2010, 51, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Korkolopoulou, P.; Angelopoulou, M.; Siakantari, M. Evaluation of DNA topoisomerase II blastoff expression provides contained prognostic information in not-Hodgkin's lymphomas. Histopathology 2001, 38, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, M. Topoisomerase II alpha—A cardinal prognostic factor in breast carcinoma. Political leader. J. Pathol. 2009, 60, 67–75. [Google Scholar]
- Burgess, D.J.; Doles, J.; Zender, 50. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 9053–9058. [Google Scholar] [CrossRef]
- Dwarakanath, B.South.; Khaitan, D.; Mathur, R. Inhibitors of topoisomerases as anticancer drugs: Problems and prospects. Indian J. Exp. Biol. 2004, 42, 649–659. [Google Scholar]
- Zijlstra, J.G.; de Jong, S.; de Vries, Due east.G.; Mulder, N.H. Topoisomerases, new targets in cancer chemotherapy. Med. Oncol. Tumor Pharmacother. 1990, seven, 11–18. [Google Scholar] [CrossRef]
Effigy i. Lymphomas of diffuse compages composed of big B cells. (a) Centroblastic subtype (DLBCL-CB) composed of compatible cells with round to oval vesicular nuclei with multiple minor nucleoli and loftier mitotic rate. Hematoxylin and eosin staining. Bar = 20µm. (b) Immunoblastic subtype (DLBCL-IB) composed of big cells with round to oval nuclei with prominent unmarried key nucleolus. Hematoxylin and eosin staining. Bar = 20µm. (c) DLBCL, centroblastic subtype with a high Ki67 index value. Indirect immunohistochemistry. Mayer's hematoxylin counterstain. Bar = 20µm. (d) Low, (e) moderate, (f) high level of TOPIIα expression in DLBCL. Indirect immunohistochemistry. Mayer's hematoxylin counterstain. Bar = twenty µm.
Figure one. Lymphomas of diffuse architecture equanimous of large B cells. (a) Centroblastic subtype (DLBCL-CB) composed of uniform cells with round to oval vesicular nuclei with multiple small nucleoli and high mitotic charge per unit. Hematoxylin and eosin staining. Bar = 20µm. (b) Immunoblastic subtype (DLBCL-IB) equanimous of big cells with round to oval nuclei with prominent single central nucleolus. Hematoxylin and eosin staining. Bar = 20µm. (c) DLBCL, centroblastic subtype with a loftier Ki67 alphabetize value. Indirect immunohistochemistry. Mayer'south hematoxylin counterstain. Bar = 20µm. (d) Low, (e) moderate, (f) high level of TOPIIα expression in DLBCL. Indirect immunohistochemistry. Mayer's hematoxylin counterstain. Bar = xx µm.
Effigy 2. The correlation between Ki67 alphabetize and mitotic count (MC). R means Spearman'southward rank correlation coefficient.
Figure ii. The correlation betwixt Ki67 alphabetize and mitotic count (MC). R means Spearman's rank correlation coefficient.
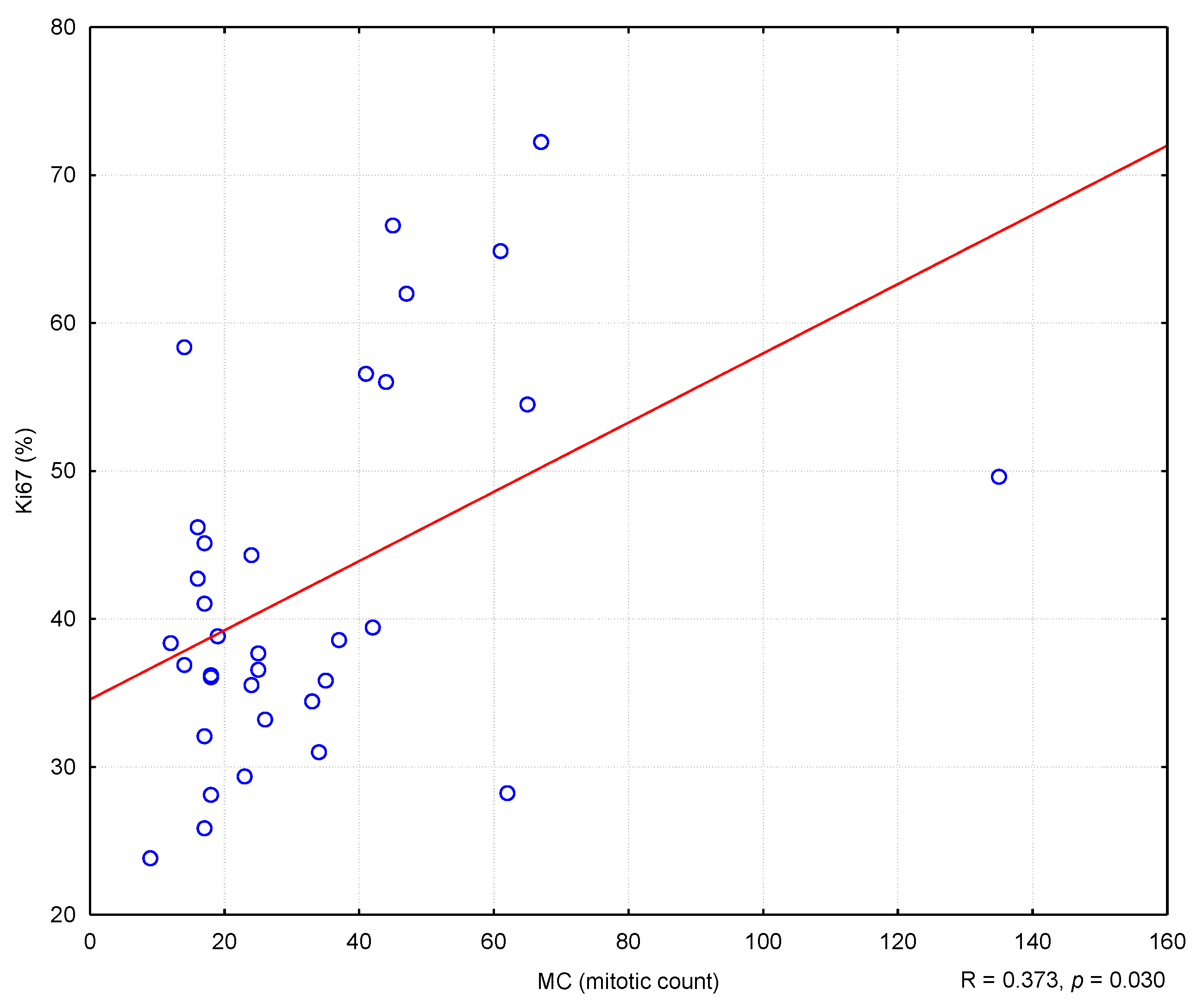
Figure three. The correlation between TOPIIα expression and mitotic count (MC). R—Spearman's rank correlation coefficient. "+" ways very low expression, "++" ways depression expression, "+++" ways medium expression, "++++" means high expression.
Figure 3. The correlation betwixt TOPIIα expression and mitotic count (MC). R—Spearman's rank correlation coefficient. "+" means very low expression, "++" ways depression expression, "+++" means medium expression, "++++" means high expression.
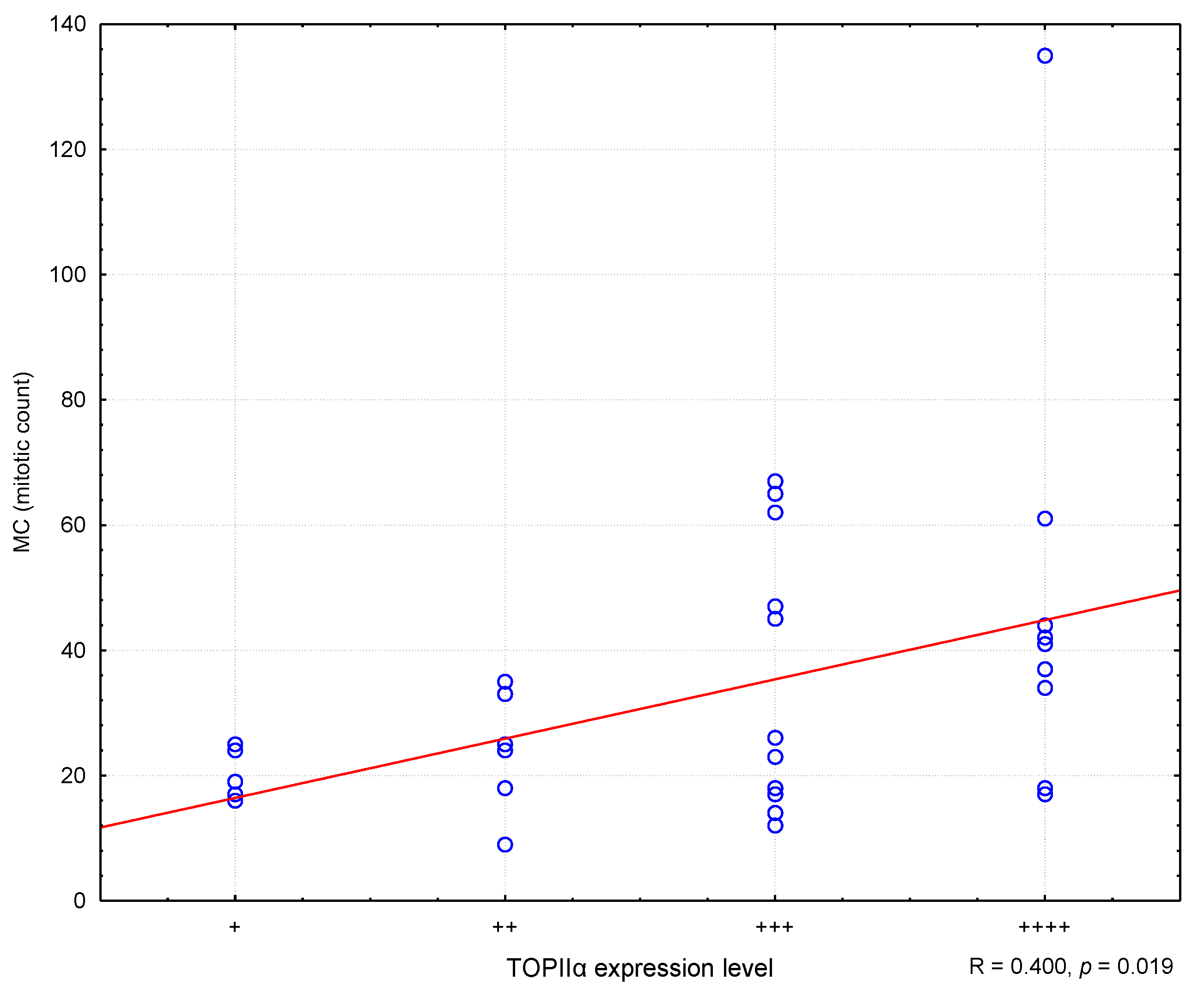
Figure four. The correlation between TOPIIα expression and Ki67. R means Spearman's rank correlation coefficient. "+" ways very low expression, "++" means low expression, "+++" ways medium expression, "++++" means high expression.
Effigy four. The correlation betwixt TOPIIα expression and Ki67. R ways Spearman'south rank correlation coefficient. "+" means very depression expression, "++" ways low expression, "+++" means medium expression, "++++" ways loftier expression.
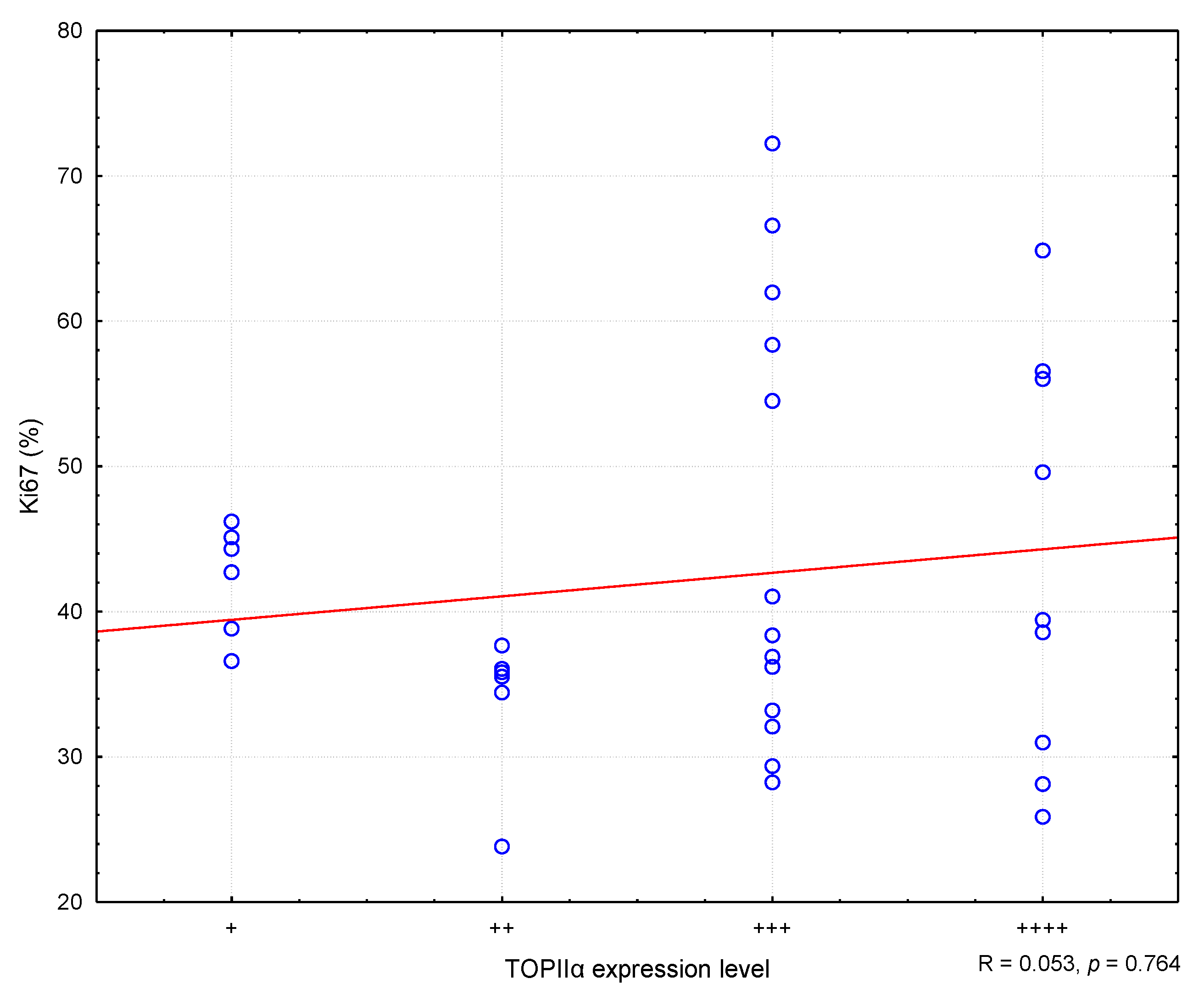
Table 1. Primary antibodies, resources, and dilution used in immunohistochemistry.
Table 1. Primary antibodies, resources, and dilution used in immunohistochemistry.
| Antibody (Anti-) | Blazon and Clone | Manufacturer | Dilution |
|---|---|---|---|
| CD3 | polyclonal, A0452 | Dako, Glostrup, Kingdom of denmark | ane:300 |
| CD79αc | monoclonal, HM57 | Dako, Glostrup, Kingdom of denmark | 1:100 |
| Ki67 | monoclonal, MIB-1 | Dako, Glostrup, Denmark | 1:100 |
| TOPIIα | monoclonal, Ki-S1 | Dako, Glostrup, Kingdom of denmark | 1:200 |
Table ii. Baseline characteristics of patient population.
Table 2. Baseline characteristics of patient population.
| Parameter | n = 34 | |
|---|---|---|
| Age | Average: 6.six (median = 6.0, range: 2.5–xiii) SD ± 2.9 | |
| Sex | Male (neutered male) | 18 (3) |
| Female (spayed female) | sixteen (9) | |
| Breed | Mixed-breed | 10 |
| High german shepherd | ix | |
| Golden retriever | 4 | |
| Boxer | ii | |
| Dog de Bordeaux | ii | |
| French bulldog | 2 | |
| Miniature schnauzer | ii | |
| Rottweiler | 2 | |
| Bernese mountain dog | 1 | |
| Clinical stage | Three | 20 |
| IV | xiv | |
Tabular array iii. Comparison of the mitotic count (MC) and the Ki67 alphabetize values depending on the type of lymphoma and TOPIIα expression.
Table 3. Comparison of the mitotic count (MC) and the Ki67 alphabetize values depending on the blazon of lymphoma and TOPIIα expression.
| Parameter | Group | north | Chiliad | Me | Min | Max | Q1 | Q3 | SD | U | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | DLBCL | 34 | 32.85 | 24.5 | 9 | 135 | 17 | 42 | 24.3 | ||
| CB | 28 | 36.82 | 29.5 | fourteen | 135 | 18.5 | 44.five | 25.04 | 11.5 | <0.001 | |
| IB | vi | fourteen.33 | 15 | 9 | 18 | 12 | 17 | 3.39 | |||
| Ki67 | DLBCL | 34 | 42.24 | 38.47 | 23.83 | 72.23 | 34.43 | 49.61 | 12.44 | ||
| CB | 28 | 42.43 | 38.12 | 25.87 | 72.23 | 33.82 | 52.06 | 12.82 | 79 | 0.843 | |
| IB | 6 | 41.35 | 41.75 | 23.83 | 58.37 | 36.2 | 46.21 | 11.57 | |||
| Ki67 | TOPIIα | –1.459 * | 0.154 | ||||||||
| expression +/++ | 12 | 38.i | 37.13 | 23.83 | 46.21 | 35.68 | 43.52 | 6.11 | |||
| expression +++/++++ | 22 | 44.5 | 39 | 25.87 | 72.23 | 32.09 | 56.56 | fourteen.45 | |||
| MC | TOPIIα | 82.5 | 0.074 | ||||||||
| expression +/++ | 12 | 21.75 | 21.5 | 9 | 35 | sixteen.5 | 25 | 7.42 | |||
| expression +++/++++ | 22 | 38.91 | 35.5 | 12 | 135 | 17 | 47 | 28.eleven | |||
Tabular array iv. Comparison of TOPIIα expression in DLBCL-CB and DLBCL-IB lymphomas.
Tabular array iv. Comparison of TOPIIα expression in DLBCL-CB and DLBCL-IB lymphomas.
| Peak II Expression Level | DLBCL | |
|---|---|---|
| CB | IB | |
| + | iv | 2 |
| fourteen.29% | 33.33% | |
| ++ | 5 | 1 |
| 17.86% | xvi.67% | |
| +++ | 10 | 3 |
| 35.71% | 50.00% | |
| ++++ | 9 | 0 |
| 32.14% | 0.00% | |
| Total | 28 | 6 |
| Mean rank | 18.63 | 12.25 |
| Statistical analysis | U = 52.5 p = 0.159 | |
| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open up access commodity distributed nether the terms and weather of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/four.0/).
Source: https://www.mdpi.com/2076-2615/11/4/1183/htm
0 Response to "Prognostic Significance of Morphotypes in Canine Lymphomas a Systematic Review of Literature"
Post a Comment